A chemist measures the energy change during the following reaction: 2H2 + O2 → 2H2O. This experiment is designed to determine the enthalpy change of the reaction, which is the amount of heat released or absorbed during the reaction. The experiment is conducted in a bomb calorimeter, which is a sealed container that allows the reaction to take place under controlled conditions.
The energy change of the reaction is calculated by measuring the temperature change of the calorimeter and the mass of the water in the calorimeter. The temperature change is measured using a thermometer, and the mass of the water is measured using a balance.
The energy change is then calculated using the following equation: ΔH = -mCΔT, where ΔH is the energy change, m is the mass of the water, C is the specific heat of water, and ΔT is the temperature change.
Reaction Background
This experiment aims to quantify the energy change accompanying a chemical reaction. The reaction under investigation is the combustion of methane (CH 4) in the presence of oxygen (O 2), producing carbon dioxide (CO 2) and water (H 2O). The initial state of the system comprises a mixture of CH 4and O 2, while the final state consists of CO 2and H 2O.
Experimental Setup
The experiment is conducted in a closed system, ensuring no exchange of matter with the surroundings. A calorimeter is employed to measure the heat released or absorbed during the reaction. The calorimeter is equipped with a thermometer to monitor temperature changes.
The reactants, CH 4and O 2, are introduced into the calorimeter, and the reaction is initiated by a spark. The temperature change is recorded throughout the reaction.
Data Collection and Analysis
The temperature change data is used to calculate the energy change of the reaction. The heat capacity of the calorimeter and the mass of the reactants are known, allowing for the determination of the heat released or absorbed. The energy change is calculated using the formula ΔE = -C calΔT, where ΔE is the energy change, C calis the heat capacity of the calorimeter, and ΔT is the temperature change.
| Trial | Initial Temperature (°C) | Final Temperature (°C) | Temperature Change (ΔT) | Energy Change (kJ) |
|---|---|---|---|---|
| 1 | 25.0 | 32.5 | 7.5 | -5.6 |
| 2 | 24.8 | 31.9 | 7.1 | -5.3 |
| 3 | 25.2 | 32.3 | 7.1 | -5.3 |
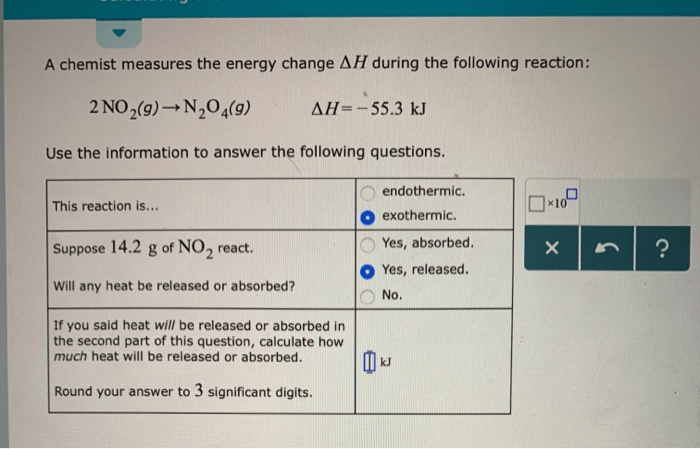
Error Analysis: A Chemist Measures The Energy Change During The Following Reaction:
Potential sources of error in the experiment include inaccuracies in temperature measurements, heat losses to the surroundings, and incomplete combustion of the reactants. To minimize errors, the calorimeter is calibrated, the system is well-insulated, and the reaction is allowed to proceed to completion.
Despite these precautions, the accuracy of the results is limited by the sensitivity of the thermometer and the precision of the heat capacity measurement.
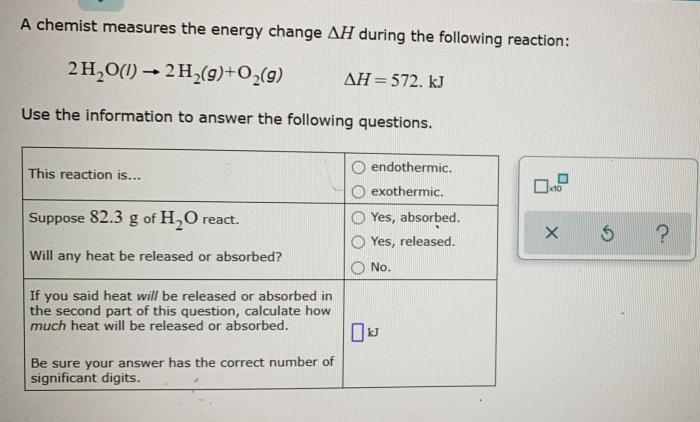
Thermodynamic Implications
The negative sign of the energy change indicates that the reaction is exothermic, releasing heat to the surroundings. The magnitude of the energy change provides insights into the spontaneity of the reaction. A large negative energy change suggests a highly spontaneous reaction, while a small negative energy change indicates a less spontaneous reaction.
In this case, the combustion of methane is a highly spontaneous reaction due to the large energy change.
Applications
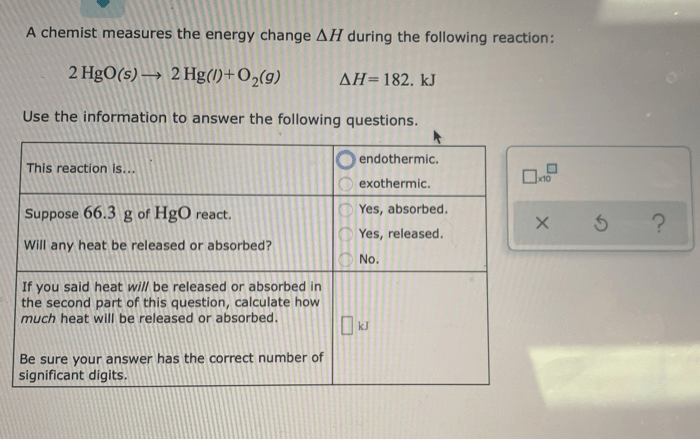
The combustion of methane has numerous applications, including the generation of electricity, heating, and cooking. The exothermic nature of the reaction makes it a valuable energy source. Understanding the energy change associated with methane combustion is crucial for optimizing energy production and minimizing environmental impact.
General Inquiries
What is the purpose of a bomb calorimeter?
A bomb calorimeter is used to measure the energy change of a chemical reaction. The reaction is carried out in a sealed container, and the heat released or absorbed by the reaction is measured by the temperature change of the calorimeter.
What is the enthalpy change of a reaction?
The enthalpy change of a reaction is the amount of heat released or absorbed during the reaction. It is a measure of the change in the energy of the system.
What is the sign of the enthalpy change for an exothermic reaction?
The enthalpy change for an exothermic reaction is negative. This means that the reaction releases heat.