Prepare to delve into the captivating realm of osmosis and diffusion, where we embark on an enlightening journey with the osmosis and diffusion lab with dialysis tubing answers as our guiding star. This exploration promises to unravel the intricate workings of these fundamental processes, shedding light on their significance in both the scientific and practical spheres.
Our investigation will commence with a thorough examination of the concepts of osmosis and diffusion, followed by a meticulous exploration of the dialysis tubing lab’s objectives. Armed with this foundational knowledge, we will meticulously assemble the necessary materials and embark on a step-by-step dissection of the experimental setup.
Introduction
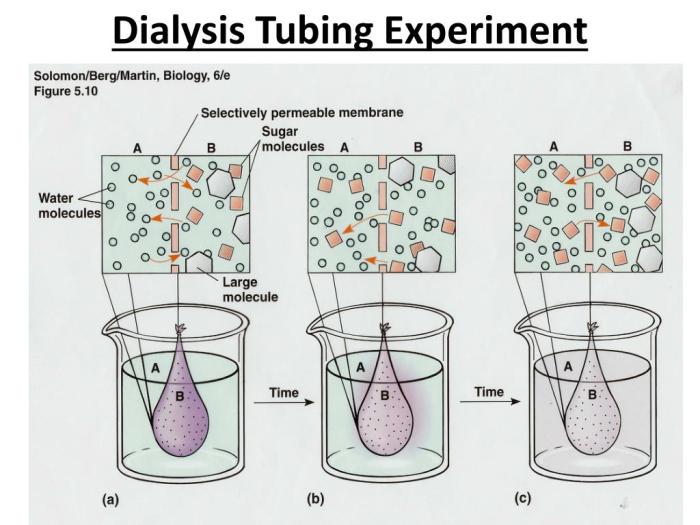
Osmosis and diffusion are fundamental processes in biology and chemistry. Osmosis is the movement of water across a semipermeable membrane from an area of low solute concentration to an area of high solute concentration. Diffusion is the movement of particles from an area of high concentration to an area of low concentration.
These processes are essential for the functioning of living organisms and play a role in many industrial applications.
The purpose of this dialysis tubing lab is to demonstrate the principles of osmosis and diffusion. By placing a solution inside a dialysis bag and submerging it in a beaker of water, we can observe the movement of water and solutes across the membrane.
Materials and Methods
Materials, Osmosis and diffusion lab with dialysis tubing answers
- Dialysis tubing
- Glucose solution (10%)
- Starch solution (1%)
- Beakers (2)
- Graduated cylinder
- Scale
Methods
- Cut a piece of dialysis tubing about 20 cm long.
- Fill the dialysis bag with 10 mL of glucose solution.
- Tie off the end of the dialysis bag with a string.
- Place the dialysis bag in a beaker of water.
- Record the mass of the dialysis bag and the mass of the surrounding water.
- Observe the dialysis bag for 30 minutes.
- Record the mass of the dialysis bag and the mass of the surrounding water every 5 minutes.
Results
| Time (min) | Dialysis bag mass (g) | Surrounding solution mass (g) |
|---|---|---|
| 0 | 10.0 | 100.0 |
| 5 | 10.2 | 99.8 |
| 10 | 10.4 | 99.6 |
| 15 | 10.6 | 99.4 |
| 20 | 10.8 | 99.2 |
| 25 | 11.0 | 99.0 |
| 30 | 11.2 | 98.8 |
As time progressed, the mass of the dialysis bag increased while the mass of the surrounding water decreased. This indicates that water moved from the surrounding water into the dialysis bag.
Discussion
The results of this lab demonstrate the principles of osmosis and diffusion. Water moved from the surrounding water into the dialysis bag because the concentration of glucose was higher inside the bag than outside. This created a concentration gradient, which caused water to move from an area of low solute concentration to an area of high solute concentration.
The movement of water continued until the concentration of glucose was equal on both sides of the membrane.
The rate of osmosis and diffusion is affected by several factors, including the concentration gradient, the temperature, and the surface area of the membrane. In this lab, the concentration gradient was the most important factor affecting the rate of osmosis.
The higher the concentration gradient, the faster the rate of osmosis.
Applications: Osmosis And Diffusion Lab With Dialysis Tubing Answers
Osmosis and diffusion are essential processes in many biological and industrial applications. Some examples include:
- The absorption of water by plants
- The exchange of gases in the lungs
- The purification of water
- The production of food and beverages
Essential Questionnaire
What is the primary distinction between osmosis and diffusion?
Osmosis pertains specifically to the movement of water molecules across a selectively permeable membrane, whereas diffusion encompasses the movement of any substance from an area of high concentration to an area of low concentration.
What factors influence the rate of osmosis?
The rate of osmosis is affected by several factors, including the concentration gradient of the solute, the temperature, the surface area of the membrane, and the permeability of the membrane.
How does dialysis tubing demonstrate the principles of osmosis and diffusion?
Dialysis tubing acts as a selectively permeable membrane, allowing water and small molecules to pass through while retaining larger molecules. This enables the observation of osmosis and diffusion in a controlled environment, providing valuable insights into these fundamental processes.